Improvement of Care & Outcome
Earlier we reported on new closed-loop insulin systems and mentioned the the ‘#WeAreNotWaiting movement’: an online community of people with diabetes who are connecting (‘looping’) their pumps and CGM devices themselves. Around the world an increasing number of people with diabetes, numbering in the thousands, are using these open-source automated insulin delivery (AID) systems and are reporting very good results in terms of glycemic control and safety. However, since these systems are set up and maintained by (caregivers of) users themselves, often using out-of-warranty components (i.e. pumps and sensors), safety, ethical and legal issues may arise:
A consensus statement was recently published by the OPEN International Healthcare Professional Network and OPEN Legal Advisory Group (including Per Winterdijk, pediatrician at Diabeter and himself using an open-source AID). The goals of this consensus statement are:
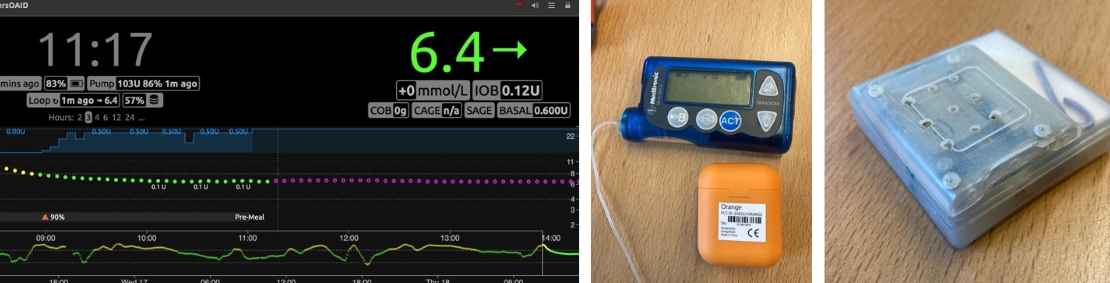
Key points:
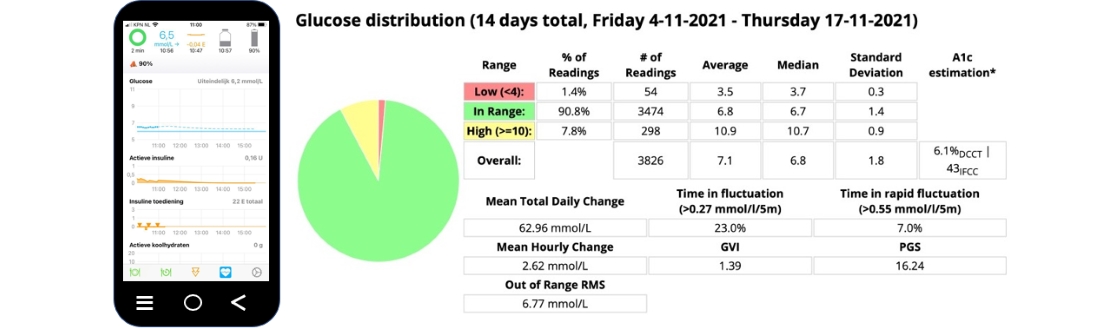
As the authors state:
Please click here for the full-text pdf.