Precision Prediction & Precision Medicine
Depending on genetic and environmental factors, many people with type 1 diabetes retain some residual β-cell function (i.e. insulin production), sometimes persisting for many years. Furthermore, residual β-cell function has been shown to correlate with better regulation and fewer complications. Since people with type 1 diabetes administer exogenous insulin, residual β-cell function cannot be assessed by measuring insulin. During the production of insulin, proinsulin is cleaved into insulin and C-peptide, which are excreted into the blood in equal levels. Also, C-peptide has a longer half-life, providing a more stable test window. Therefore, measuring stimulated C-peptide production is commonly used as proxy for residual β-cell function. It is becoming increasingly clear that even very low levels of C-peptide (i.e. insuline production) are associated with better outcomes and C-peptide assays are increasingly used near their detection limits.
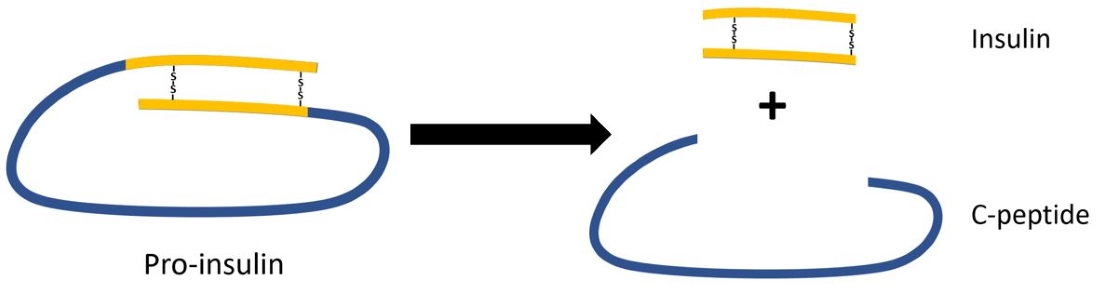
To measure serum C-peptide a wide range of assays are currently available. For the Biomarker project we considered to switch from the immunoradiometric (IRMA) assay by Beckman, used in our clinical laboratory (IJsselland Hospital) to an ultrasensitive enzyme-linked immunosorbent (ELISA) assay by Mercodia, which should have a lower detection limit according to the manufacturer. The two assays were compared in verification experiments with samples from the Biomarker project.
Key findings:

Concluding, the authors state
Please click here for the pdf file.